
MAY 2023 – NEW TESTING TECHNIQUE for Allergies & Sensitivities!
PAX by NEXTMUNE
https://insights.nextmune.com/pax/petallergyxplorer
Molecular allergology is a state-of-the-art approach to the detection of sensitisations, whereby defined single allergen components are used for the determination of specific IgE in place of traditionally-used allergen extracts. The molecular components are purified or recombinant proteins that provide a higher level of standardisation than allergen extracts and enable a more precise identification of IgE sensitisations. Pet Allergy Xplorer (PAX) is the first commercial serological IgE-specific test that uses allergen extracts and molecular components to identify which allergens are affecting pets.
THE PROBLEM
Traditionally, allergy testing in veterinary medicine involves placing an allergen extract on an enzyme linked immunoassay (ELISA) plate to incubate the serum and then administering a reagent that recognizes immunoglobin E (IgE). The resultant color reaction indicates how much IgE is present. This technique, which is used by veterinary laboratories world-wide, has not changed for decades. However, results can vary considerably, depending on the extract used, and false negatives can occur if clinically relevant protein allergen concentrations are not sufficient. For example, the extract for the house dust mite, a common pet allergen, is made by grinding the mite, adding solvents to release the allergenic proteins, and purifying the proteins.
The house dust mite contains more than 10,000 proteins, but only about 40 that cause an allergic reaction are recognized. This means a low percentage of the allergy-causing proteins are seen when an extract is evaluated, especially if a pet has a low IgE level against a particular allergen. This can easily result in a false negative. In addition, extracts can vary not just between laboratories but also allergenic extract, making results hard to reproduce.
THE SOLUTION
To gain more accurate and sensitive information, tests are needed to identify each individual allergenic protein. Instead of testing for the house dust mite (or any particular allergen) as a whole, techniques are needed to test for the specific proteins that cause an allergic reaction. Human practitioners use molecular allergology to determine the allergens causing problems to provide their patients with a better level of care.
Macro Array Diagnostics launched the Allergy Explorer (ALEX), which provides a sensitization profile for human patients based on a test panel composed of allergen extracts and molecular allergens. Since their founding in 2016, the company has launched two generations of ALEX, offering a panel that covers nearly 100% of the world’s relevant allergens. It also developed the Food Xplorer (FOX), to detect IgG-mediated food intolerances.
March 2021
Previously the only accurate way to test for IBD was via an invasive scope.
(Updated October 5, 2022) Thanks to recent technology, they now have non-invasive ways to test for IBD indication. A Simple Blood Test.
Currently there is one lab that is now doing blood testing for IBD… previously Antech also had a blood test for IBD but Antech pulled it off the market as it was not accurate.
- TAMU: Canine C-reactive Protein – Gastrointestinal Laboratory (tamu.edu)
- And if you want to do a saliva test for what the sensitivities might be, check out Dr. Jean Dodds Sensitivity Test:
- DR. JEAN DODDS: Nutriscan | Hemopet
The following is an excellent comprehensive overview of IBD Inflammatory Bowel Disease- IBD | Long Beach Animal Hospital (lbah.com)
July 2022 Inflammation Protein Protectant Identified
https://news.cornell.edu/stories/2022/07/scientists-identify-key-molecular-protector-gut-health
Study identifies a protein that plays key role in protecting the gastrointestinal tract from inflammation
A protein called Zbtb46, expressed by specialized immune cells, has a major role in protecting the gastrointestinal tract from excessive inflammation, according to a study from researchers at Weill Cornell Medicine.
The finding, which appears July 13 in Nature, is a significant advance in the understanding of how the gut maintains health and regulates inflammation, which could lead to better strategies for treating diseases like inflammatory bowel disease (IBD).
“We’ve known that there are related families of immune cells in the gut that can either protect from inflammation or at other times be major drivers of inflammation,” said senior author Dr. Gregory Sonnenberg, associate professor of microbiology and immunology in medicine in the Division of Gastroenterology & Hepatology, and a member of the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine. “This new finding helps us understand how these cells are regulated to optimally promote intestinal health and prevent inflammation.”
IBD, which includes Crohn’s disease and ulcerative colitis, affects several million people in the United States. These chronic inflammatory disorders target the gut, can be seriously debilitating, and treatments may not work well for some patients—mainly because scientists don’t have a complete picture of what is driving these diseases and how the sophisticated immune cell networks in the gut support tissue health.
Dr. Sonnenberg’s laboratory has been advancing the science of gut immunity with studies of recently identified immune cells called ILC3s. These “innate lymphoid cells” are related to T cells and B cells, and clearly have important roles in protecting the gut and other organs from excess inflammation. However, in the context of IBD or colorectal cancer they become altered. In general, scientists have wanted to know more about how these cells work.
To this end, Dr. Sonnenberg and his team, including first author Dr. Wenqing Zhou, a postdoctoral researcher in the Sonnenberg Laboratory, set out to make a detailed catalog of ILC3s and other related immune cells residing in the large intestine of mice, using relatively new single-cell sequencing techniques.
A surprise finding was that a subset of ILC3s express Zbtb46, an anti-inflammatory protein that prior studies had suggested is produced only in dendritic cells, a very different type of immune cell. The researchers showed in experiments with mice that ILC3s expressing Zbtb46 have a strong ability to restrain inflammation following gut infection. When they blocked Zbtb46 expression in ILC3s, gut infection led to signs of severe inflammation, including a rise in the numbers of other gut immune cells promoting inflammation.
“This demonstrates a major role for this new pathway in responding to inflammation in the intestine of children with IBD, suggesting it could represent a novel therapeutic target,” Dr. Sockolow said.
Finally, the scientists found that Zbtb46-expressing ILC3s are also the main producers in the gut of a cytokine, IL-22, that protects against bacterial infection. Mice without the entire population of Zbtb46-expressing ILC3s are more susceptible to intestinal inflammation induced by gut infection.
On the whole, our findings suggest a critical function of ILC3-intrinsic Zbtb46 in restraining gut inflammation, and a non-redundant role for Zbtb46-expressing ILC3s in protecting the gut following enteric infection.”
Dr. Wenqing Zhou, First Author
The next steps for Dr. Sonnenberg, Dr. Zhou and their colleagues will include studies to determine precisely how Zbtb46—a transcription factor protein that binds to DNA to control other genes’ expression levels—exerts its anti-inflammatory effects. Ultimately, they hope, future drugs that boost Zbtb46-related anti-inflammatory signaling could help treat IBD patients.
Dr. Sonnenberg added that, in principle, drugs that block Zbtb46-related signaling might be useful in situations, including some cancers, where enhancing immune activity is desirable. This is an active area of investigation in his laboratory.
Zhou, W., et al. (2022) ZBTB46 defines and regulates ILC3s that protect the intestine. Nature. doi.org/10.1038/s41586-022-04934-4.
CYTOPOINT AS A POSSIBLE TREATMENT FOR IBD ??? … MAYBE.
Might be worth asking your vet if this could benefit your dog if struggling with IBD…..
IBD and Food Allergies (and/or Food Sensitivities)

December 2018
We at Epi4Dogs often hone in on food allergies or food sensitivities in relation to IBD along with SID/dysbiosis. HOWEVER….. we all may be better served by broadening our understanding of this. Not all food sensitivities/allergies have the same trigger.
Here is a nice chart, by Dr. J.M. Craig, of: Re-Fur-All Referrals, Newbury, Berkshire, UK, published in the Journal of Small Animal Practice, (2018), DOI: 10.1111/jsap.12959, Accepted: 6 October ……….. that summarizes food sensitivities/allergies possibilities:
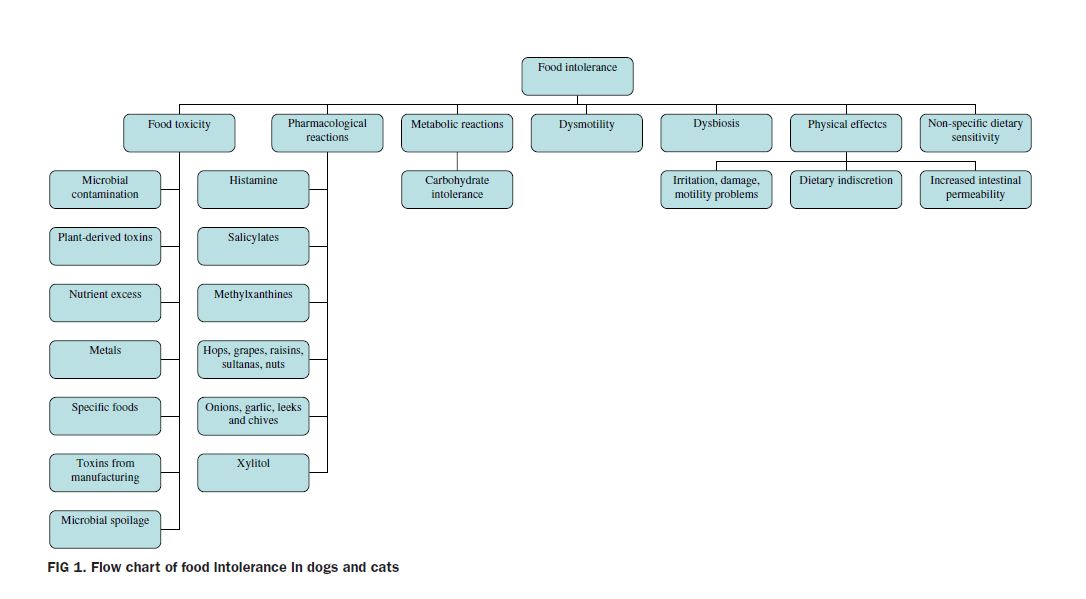
October 2018 : Here is an EXCELLENT article by Dr. J.M. Craig explaining the various types of food intolerance/allergies in dogs and cats…
By identifying the trigger, it may help us more effectively treat the sensitivity/allergy.

July 2018 – The following is genetic research done with 190 GSDs (German Shepherd Dogs) with IBD and identified various alleles:
The following are various gastrointestinal conditions that some times accompany EPI. Understanding how some of these other conditions are treated
may help those that are dealing with more than just EPI.
Links to recent allergy research reports:
http://www.nvma.org/assets/site/Proceeding_Files/January%202015%20Proceedings.pdf
http://www.veterinarypracticenews.com/Sponsor-Content/?prx_t=6CkCAKusGAN3wFA
In addition to the below articles…. more and more folks dealing with not food allergies but rather food sensitivities are finding success with Dr. Dodd’s NutriScan Food test… to help them identify the sensitivities. Sensitivities are different from Allergies.. If you are interested in learning more about NutriScan, please click here:
IBD & Food Allergies presentation by:
The British Veterinarian Association Congress 2007
Link provided with permission of the BVA
http://www.bva.co.uk/public/documents/07congress_food_allergies_watson_pp.pdf
Dietary management of intestinal disease
Stanley L. Marks
BVSc, PhD, Dipl ACVIM (Internal Medicine, Oncology), Dipl ACVN, California, USA
www.ivis.org
International Congress of the Italian Association of Companion
Animal Veterinarians
May 30 – June 1 2008
Rimini, Italy
The disciplines of nutrition and gastroenterology are intimately elated by virtue of the primary role played by the astrointestinal tract in the assimilation of food. The therapeutic pproach to most gastrointestinal diseases involves a ombination of pharmacologic and nutritional therapy. nfortunately, the beneficial impact of nutritional therapy is ften ignored in many patients, resulting in incomplete or elayed resolution of signs. Restriction or manipulation of ndividual dietary components is perhaps the single most mportant factor in the treatment of either acute or chronic astrointestinal disturbances. Despite these recommendations, here is a paucity of information pertaining to the utritional requirements of dogs and cats with gastrointestinal isorders. This presentation will focus on the dietary management of chronic small- and large-bowel disease, management of exocrine pancreatic disease, and management f hepatic disease.
CHRONIC SMALL-BOWEL DISEASE
Dietary modification is essential for the management of ost patients with chronic small-bowel disease. Dogs with diarrhea associated with small-bowel disease should be
managed with a diet that is highly digestible, moderately at-restricted, lactose-free, gluten-free, and “hypoallergenic”. he theoretical concerns with dietary fibers “abrasive” ffects on the inflamed intestinal tract and the presumed egative effects of fiber on small intestinal assimilation of utrients should be reconsidered because the gelling and inding properties of fiber may be beneficial in certain mall intestinal diseases.1 Less information is known about he nutritional recommendations for the management of hronic diarrhea associated with feline small-bowel disease. n contrast to dogs, cats with small-bowel disease seem to tolerate diets containing higher levels of fat,2 and high fat iets (79% fat calories) do not appear to delay gastric emptying n the cat.3
Dietary Fat
A fat-restricted diet is important in the management of a variety of gastrointestinal diseases in dogs, even though fat is a valuable caloric source and enhances the palatability of the diet. Fat delays gastric emptying,4,5 and fat-restricted diets appear to be better tolerated in a variety of gastrointestinal diseases. The assimilation of dietary fat is a relatively complex process and malabsorbed fatty acids are hydroxylated by intestinal and colonic bacteria. These hydroxy-fatty acids stimulate colonic water secretion and exacerbate diarrhea and fluid loss.6 Fat malassimilation can also be associated with malabsorption of bile acids, resulting in deconjugation of unabsorbed bile acids and increased mucosal permeability and secretion.7
Dietary Lactose and Gluten
Intestinal disease frequently destroys or reduces mucosal brush border enzyme activity, particularly lactase, the most superficial enzyme. Milk or other lactose-containing substances should therefore be avoided in patients with enteric disease. Failure to digest lactose results in bacterial degradation of the sugar to volatile fatty acids which can cause an osmotic diarrhea. The use of yogurt for therapy of chronic diarrhea is not recommended because of its lactose content. In addition, orally administered bacteria in yogurt do not colonize the bowel and displace the “unfavorable” microorganisms in both normal and diseased intestines. Gluten is a
component of wheat, oats, barley, and rye, all of which should be avoided in patients with inflammatory bowel disease (IBD) in the event that the diarrhea is due to a gluten enteropathy.
Dietary Protein
Adverse reactions to dietary staples are common in cats and dogs with chronic gastrointestinal disease, and can often be successfully managed by feeding selected-protein diets.8-11 Because antigenic determinants on proteins are incriminated as the precipitating factor in many cases of IBD, it is usually recommended to feed an elimination diet that is generally free of additives and preservatives, and contains a single, novel protein source that is highly digestible, or a hypoallergenic formula.12 There are no protein sources that are inherently hypoallergenic. The protein source should be highly digestible because intact proteins are far more antigenic
than polypeptides and amino acids.13
INFLAMMATORY BOWEL DISEASE (IBD)
The inflammatory bowel diseases (IBD) are the most common causes of chronic vomiting and diarrhea in dogs, and refer to a group of idiopathic, chronic gastrointestinal tract disorders, characterized by infiltration of the lamina propria by lymphocytes, plasma cells, eosinophils, macrophages, neutrophils, or combinations of these cells.12 The diagnosis of IBD requires the comprehensive exclusion of potential causes of gastrointestinal inflammation, including intestinal parasites, small intestinal bacterial overgrowth, bacterial enterocolitis, dietary intolerances or allergies, and neoplasia.12 Failure to eliminate known causes of gastrointestinal
inflammation which can mimic IBD can result in frustration for the owner and clinician due to poor responsiveness of the animal to dietary or pharmacologic therapy.
Although the etiology of canine IBD is poorly understood, most of the evidence for proposed causes in dogs have been extrapolated from humans with ulcerative colitis and rohn’s disease.13-17 Caution should be heeded in making extrapolations across species, because human and canine BD are not synonymous. Proposed causes for human IBD include defective immunoregulation of the gut-associated lymphoid tissue that may be precipitated by permeability defects,14 infectious and parasitic agents,15,16 and dietary allergies.13,17 There is provocative evidence from clinical observations and animal models to incriminate normal luminal bacteria or bacterial products in the initiation and perpetuation of canine IBD.18,19 The clinical response to hypoallergenic or elimination diets suggest that dietary factors may
influence the pathogenesis of canine IBD.8-11 The term “hypoallergenic” refers to a diet that is generally free of additives and preservatives, and contains a hydrolyzed protein source.
Because the presumed pathogenesis of canine IBD involves hypersensitivity to luminal dietary or microbial antigens, therapy is aimed at removing any antigenic source of inflammation, 13,18,19 followed by suppression of the cell-mediated inflammatory response in the gastrointestinal tract. Unfortunately, the increased utilization of commercial lamb-based formulas has diminished its application in many elimination diets, necessitating the selection of more “exotic” protein sources such as kangaroo, ostrich, rabbit, and venison. It is important that the ingredients list of a potentially hypoallergenic diet be thoroughly evaluated, because diets with several protein sources (lamb, beef, rice, and wheat) are commonly marketed with a claim to hypoallergenicity. All flavored vitamins and flavored heartworm preventatives, table
scraps, and raw-hide chews should be avoided during the feeding of the controlled diet. The concept of feeding a “sacrificial protein source” during the early phase of therapy is currently under investigation to minimize the likelihood of the animal becoming sensitive to the novel protein source while the intestine is still inflamed and more permeable to indigestible dietary mroteins.12 The first novel protein offered is referred to as a sacrificial protein because it is introduced while the gut mucosal barrier is abnormally permeable, increasing the likelihood of the patient acquiring an allergy to this protein. The sacrificial protein is fed for approximately 6 weeks, after which time a second novel protein source is offered. This diet change would coincide with the lowering of the prednisone dose from the immunosuppressive to the anti inflammatory range. There is no data advocating the benefits of this dietary concept over the implementation of a rotational dietary approach in which two diets containing novel protein sources are alternatively fed e very 3 to 5 days. Likewise, there is no documented benefit of either of the two previously mentioned dietary approaches to the feeding of a single novel protein source diet that is fed until the patient becomes intolerant to the protein source. A small percentage of dogs with severe IBD will fail to respond to elimination diets containing novel, intact protein sources despite appropriate pharmacologic therapy. These patients may benefit from diets containing hydrolyzed protein sources in which the molecular weight of the polypeptide molecule is below 18,000 daltons (Royal Canin Hypoallergenic formula) or from home-cooked diets containing single novel protein and carbohydrate sources.
CHRONIC LARGE-BOWEL DISEASE
Dietary recommendations for the management of diarrhea associated with large-bowel disease is controversial, because the veterinary information is often derived from few data-based refereed publications and many uncontrolled clinical observations. The response to dietary therapy can vary dramatically from one patient to another, with some animals showing improvement on low residue, elimination diets, 9,20,21 and others improving on less digestible diets containing soluble or insoluble fiber sources.22 Commercial diets that are viable for the management of large bowel disease in dogs include Royal Canin Intestinal formula, Royal Canin Sensitivity Control, or Royal Canin Diabetic formula. The latter formula contains increased amounts of fermentable and non-fermentable fiber and moderate amounts of fat.
Dietary Protein
There is evidence to suggest that some forms of colitis may be associated with a dietary sensitivity similar to that observed with small bowel disease.8 Proteins, lipoproteins, glycoproteins, lipopolysaccharides, and carbohydrates can induce an immunologic or inflammatory response similar to that observed in the small intestine. The theoretical benefit for utilizing highly digestible “hypoallergenic” diets for patients with colitis includes reducing the digestive challenge to the large intestine and minimizing the likelihood of dietary antigens actually reaching the colon, thus lessening the likelihood of an immunological reaction.21 Several studies in the veterinary literature suggest that some patients may benefit from diets providing novel, highly digestible protein sources.9-11 One prospective study reported a resolution in clinical signs associated with idiopathic chronic colitis in 13 dogs fed rice and cottage cheese. Only 2 of the dogs in this study tolerated a challenge with the original commercial diet that had been fed at the time of the onset of signs of colitis. A second prospective study reported resolution of clinical signs associated with
lymphocytic-plasmacytic colitis in 6 cats fed lamb and rice, or horsemeat.10 Four of those cats were successfully placed on a veterinary therapeutic diet after two weeks on the elimination diet. Subsequent reintroduction of a feline commercial diet resulted in recurrence of diarrhea in 3 cats, which resolved after the diet was removed. In a third prospective study, 20 dogs with a non-seasonal, pruritic skin disorder and gastrointestinal signs were placed on one of two novel protein diets; a homemade diet of fish and potato or a commercial diet containing fish and soy.11 Gastrointestinal signs were reduced or eliminated while the dogs were on their dietary treatments. Recurrence of gastrointestinal signs was seen concurrently with a recurrence of pruritus when the dogs were challenged with components of their original diets. The challenge results in these three studies strongly suggest a dietary role in the pathogenesis of this disorder and also illustrate the potential importance of dietary therapy. Highly digestible commercial diets, without novel protein sources, have also been shown to be effective in the management
of patients with large-bowel diarrhea. In one prospective study, 11 dogs with idiopathic, chronic colitis were dreated for 4 months with a commercial restricted antigen diet containing protein sources limited to chicken and rice.21 All dogs were simultaneously treated with sulfasalazine (20 to 40 mg/kg/day). Previous dietary management had been attempted in 9 of the 11 dogs, but diet histories were not provided. Within 1 month of consuming the limited antigen diet, 60% of the dogs required no sulfasalazine, or a reduced dosage than when originally presented. Within 2 months, 90% were stabilized with no drug therapy. In this study it was difficult to differentiate between the dietary and drugrelated dffects of management because the two were administered simultaneously. The authors also suggest it was likely that both the digestibility (although not determined in the study) and the limited allergen content of the diet were important factors that may have contributed to the successful management of the dogs. A recent study investigated the prevalence of adverse reactions to foods in cats with chronic gastrointestinal problems. 8 The diagnosis of food sensitivity was made by dietary elimination-challenge studies using commercial selected-protein diets (chicken or venison-based). Sixteen (29%) of the 55 cats with chronic idiopathic gastrointestinal problems were diagnosed as food sensitive. The clinical signs of another 11 cats (20%) resolved on the elimination diet but did not recur after a challenge with their previous diet. The most common allergens identified were beef, wheat and corn gluten. Weight loss occurred in 11 of tthe affected cats and large-bowel diarrhea was more common than small-bowel diarrhea. The clinical feature most suggestive of food sensitivity was concurrent occurrence of gastrointestinal and dermatological signs. Collectively, 50% of the cats fed the selected-protein diets had resolution of their clinical signs. This observation suggests that selected-protein diets should be considered an important part of the management of cats with chronic idiopathic gastrointestinal disease.
Dietary Fiber
High fiber diets containing soluble, insoluble or mixed fiber are frequently recommended for the treatment of chronic colitis. The use of soluble (fermentable) fiber in preference to insoluble (non-fermentable) fiber is sometimes advocated because most soluble fibers generate butyrate, the principle source of energy for the colonocyte, and other short-chain fatty acids.23 Short-chain fatty acids may lower the colonic luminal pH, impeding the growth of pathogens.23 The use of dietary fiber can have deleterious consequences. As dietary fiber increases, digestibility of essential nutrients decreases, which may result in nutritional imbalances, particularly
if a marginal quality diet is being fed. Fructooligosaccharides (FOS) are carbohydrates that resist digestion by the enzymes in the gastrointestinal tract and can be metabolized by the microbial species that colonize the distal small intestine and colon. The addition of FOS to feline diets at 0.75% (DM) did not affect duodenal flora,
but it did increase the numbers of lactobacilli and reduce the numbers of E. coli in the fecal flora of healthy cats.24,25 Healthy German shepherds believed to have bacterial overgrowth were supplemented with FOS at 1.0% (AF) of their diet.26 Changes were recognized in the duodenal bacterial flora but these changes were of less magnitude than seen in normal dogs for these parameters. The clinical significance of these studies in cats and dogs with colitis is unknown. Recently, treatment of chronic idiopathic large bowel diarrhea with a highly digestible diet and soluble fiber was reviewed in a retrospective study of 37 dogs.27 Treatment with a soluble fiber source (Metamucil), added to a highly digestible diet, resulted in a very good to excellent response in 23 of the 27 dogs that received supplementation. Dogs
classified as having a very good or excellent response to soluble fiber supplementation received no other additional therapy except for occasional loperamide or diphenoxylate. Fiber supplementation was later reduced or eliminated in 11 dogs; diarrhea returned in 6 of them.
Polyunsaturated Fatty Acids
Manipulation of the dietary ratio of omega-6 to omega-3 polyunsaturated fatty acids (PUFA’s) has the potential to reduce the inflammatory response in human ulcerative colitis and Crohn’s disease patients.28,29 Diets enriched in n-3 fatty acids can result in the incorporation of the n-3 fatty acids into biological membranes, with a corresponding decrease in concentrations of the proinflammatory n-6 fatty acids such as arachidonic acid (20:4,n-6). The therapeutic dotential of dietary precursor modulation by a fish-oil-supplemented diet (n-3 fatty acids), such as eicosapentaenoic acid (C20:5,n-3) and docosahexaenoic acid (C22:6,n-3) in
the therapy of ulcerative colitis has been shown to result in a 35% to 50% decrease in neutrophil production of LTB4.28 Significant improvement in symptoms and histologic appearance of the rectal mucosa has been observed in several small series of patients with Crohn’s disease and ulcerative colitis given fish oil at 3 to 4 g daily for 2 to 6 months in uncontrolled studies.29 However, a larger, randomized, double-blind trial comprising 96 patients with ulcerative colitis failed to reveal any benefit in remission maintenance or treatment of relapse on 4.5 g of eicosapentaenoic acid daily, despite a significant reduction in LTB4 synthesis by blood peripheral polymorphonuclear cells.30 It should be emphasized, however, that the anti-inflammatory actions of the fish oils, in addition to inhibition of LTB4, include suppression of IL-1 and platelet activating factor synthesis and scavenging of free oxygen radicals.30 The impact of increased lipid peroxidation after fish oil supplementation should be considered when altering the n-6:n-3 fatty acid ratio.31 Antioxidant supplementation may be able to counteract the potentially adverse effects of n-3 fatty
acids. There are no reports in the veterinary literature demonstrating the efficacy of n-3 fatty acid supplementation in managing canine or feline large intestinal disease. Studies in healthy dogs fed diets with n-6:n-3 fatty acid ratios of 5:1 and 10:1 demonstrated a decreased production of LTB4 in plasma, neutrophils and skin.32 Increases in certain long chain n-3 fatty acids and decreases in arachidonic acid were identified in the small intestine and colonic mucosa of healthy Beagles fed the same ratios.33 Further research is necessary to establish a dosage of PUFA’s and to determine the clinical benefits in dogs and cats with large bowel diseases.
DIET SELECTION FOR LARGE-BOWEL DISEASE
The optimal nutritional approach for the management of large-bowel disease remains to be determined and varies from animal to animal. Three types of foods are frequently used in the management of large-bowel diarrhea; 1) highly digestible, low-residue foods, 2) fiber-enhanced foods, and 3) elimination or novel foods. One common approach is to feed a complete and balanced commercial diet containing moderate amounts of a highly digestible protein source to which the animal has not been previously exposed with moderate levels of dietary fat (12-15% or 15-20% DM for dogs and cats, respectively). There are a number of commercial diets available that meet these specifications. The supplementation of fermentable fiber sources such as psyllium or oat bran may be necessary in animals showing partial resolution of their clinical signs. Failure to respond to these recommendations may necessitate selecting another novel protein source diet, adding insoluble fiber to the diet, or further dietary fat restriction. A complete and balanced computer-generated homemade diet that is prepared by a veterinary nutritionist is a viable alternative for dogs and cats that do not show improvement with conventional dietary recommendations.
PANCREATITIS
The traditional recommendation for managing dogs with pancreatitis is to give nothing by mouth for 2 to 3 days, followed by the gradual introduction of water and a fat-restricted diet such as cottage cheese and rice or Royal Canin Digestive LF formula. Fluid and electrolyte balance is maintined with crystalloids (usually lactated Ringer’s solution) and colloid solutions such as dextran 70 or hetastarch are utilized to maintain oncotic pressure and help ensure adequate perfusion to the inflamed pancreas. Consumption of plasma protease inhibitors and saturation of available α2- macroglobulin by activated proteases is rapidly followed by acute disseminated intravascular coagulation, shock and death.34,35 Although a clinical trial in humans has failed to show the beneficial effects of fresh-frozen plasma directed at replenishing α2-macroglobulin stores, there is anecdotal evidence of its benefit in dogs with pancreatitis.36 Transfusion of fresh frozen plasma (10 – 20 ml/kg) to replace natural protease inhibitors such as α2-macroglobulin is frequently associated with amelioration of the deleterious effects associated with inflammatory mediators and activated proteases. Dietary amino acids and fatty acids are the most potent stimulators of pancreatic enzyme secretion and are thus avoided during the initial recovery period. Small amounts of water or ice cubes should be offered after the patient has stopped vomiting. If there is no recurrence of clinical signs, a diet rich in carbohydrate (rice, pasta, potatoes) nd restricted in fat and protein should be gradually reintroduced. With continued clinical improvement, gradual introduction of a fat-restricted maintenance diet should be attempted. Patients with relapsing pancreatitis or severe necrotizing pancreatitis require prolonged hospitalization and attention to their nutritional status. Patients with prolonged anorexia may require enteral feeding via jejunostomy tube or total parenteral nutrition to maintain their
metabolizable energy requirements. The clinical picture and nutritional recommendations for cats with pancreatitis differs markedly from that in dogs.
EXOCRINE PANCREATIC INSUFFICIENCY (EPI)
Nutrient malabsorption in EPI arises as a consequence of failure of intraluminal digestion, and impaired function of intestinal mucosal enzymes. Most dogs and cats with EPI can be managed with dietary modification and pancreatic enzyme supplementation. A suboptimal response to enzyme supplementation usually reflects associated small intestinal disease or bacterial overgrowth.
Diet
Fat absorption does not return to normal despite appropriate enzyme replacement therapy in dogs with EPI.39 Patients usually compensate by increasing their caloric intake, necessitating an increase of approximately 20% above their calculated maintenance requirements. Although fecal fat decreases when a fat-restricted diet is fed, excessive dietary fat restriction could decrease the absorption of fat, fat-soluble vitamins, essential fatty acids, and cholesterol. It has also been shown that a fat-restricted diet does not ameliorate signs of EPI.40 In fact, the feeding of a high-fat and high-protein diet in combination with porcine-lipase maximized fat
absorption in one experimental study in dogs with EPI.41 Studies in human patients also reveal that certain fiber sources (e.g., wheat bran, pectin) impair pancreatic enzyme activity, therefore, high-fiber diets should be avoided.42 Most dogs with exocrine pancreatic insufficiency do well when fed regular commercial maintenance diets. Patients exhibiting poor weight gain may benefit from dietary supplementationwith medium-chain triglycerides, although studies are needed to confirm whether dietary fats containing medium-chain triglycerides are directly absorbed into the portal circulation, and whether digestion by lipase with incorporation into cchylomicrons is circumvented. Fat absorption will not be improved by pre-incubation of the food with pancreatic enzymes, administration of antacids, or by addition of bile salts.
Enzyme replacement
Many different preparations of pancreatic enzymes are commercially available; however, powdered formulations have been shown to be most effective in dogs.39 Tablets, capsules and enteric-coated preparations are less effective than powdered extracts and are not recommended.43 Enzyme replacement using an initial dose of 1 teaspoonful of powdered non-enteric-coated pancreatic extract with each meal per 10 kg of body weight is generally effective for dogs, whereas cats should receive one teaspoon per meal. Animals that do not show an optimum response to this dose do not usually benefit by increasing the amount of extract. The
extract should be mixed with food immediately prior to feeding. Two meals a day are usually sufficient to promote weight gain of 0.5 to 1.0 kg per week in larger dogs; diarrhea generally resolves within 2-3 days, and coprophagia and polyphagia also often disappear within a few days. As soon as clinical improvement is apparent owners can determine a minimum effective dose of enzyme supplement that prevents return of clinical signs. This varies slightly between different enzyme preparations, and also from dog to dog. Most affected animals need at least 1 teaspoonful of extract with each peal. An economical alternative to commercially available enzyme preparations is chopped raw bovine or porcine pancreas (3 to 4 ounces per meal for a 20 kg dog) given with the food. Porcine pancreas is as effective as bovine pancreas, but there is a potential risk of infection with Aujesky’s disease since the pancreas must be fed raw. Fresh pancreas can be stored frozen for at least 3 months without loss of enzyme activity. The owner should be reminded about maintaining proper kitchen hygiene to decrease the risk of possible transmission of zoonotic diseases such as salmonellosis. There is no difference in the therapeutic response between dogs that are treated with pancreatic powder or raw pancreas.44
Vitamin Supplementation
Serum concentrations of cobalamin (vitamin B12) and vitamin E are often subnormal in dogs with EPI and do not necessarily increase in response to treatment with enzymes, even though the clinical response may otherwise be excellent. Vitamin E should be supplemented at a daily dose of 250 to 500 mg alpha-tocopherol (vitamin E) given in the food for 1 month. Low serum cobalamin concentrations in dogs have been associated with exocrine pancreatic insufficiency, severe intestinal disease, and putatively small intestinal bacterial overgrowth.45-47 Cobalamin is an essential cofactor for the activity of methylmalonyl-CoA mutase and methionine synthase.48 Anemia with hypoplastic erythropoeiticcenters in the bone marrow has been described as a consequence of cobalamin deficiency in the dog. Serum cobalamin can be assayed and if decreased, administered cubcutaneously at a dose of 500 μg per dog once weekly for 6 weeks, with the dosing schedule decreased to once every 6 to 12 months depending on serum cobalamin concentrations. Cats appear highly susceptible to cobalamin deficiency, partly as a result of the very rapid turnover of this vitamin compared with humans.49 Cats with decreased serum cobalamin concentrations should be supplemented with subcutaneously administered cobalamin at a dose of 1000 μg per cat once weekly for 6 weeks, with reassessment of the serum cobalamin concentration approximately one month after discontinuing therapy. Cases of vitamin K deficiency-responsive coagulopathies have occasionally been documented in dogs and cats with EPI and severe IBD. Parenteral vitamin K1 (2.5 mg/kg) followed by oral vitamin K1 at 0.25 to 2.5 mg/kg q12 hours should be given when there is clinical or laboratory evidence of a coagulopathy.

Antibiotics
The need for antibiotic therapy varies from patient to patient, but is usually indicated in dogs with small intestinal bacterial overgrowth. Secondary SIBO can cause diarrhea, weight loss, and malabsorption. Pancreatic enzyme supplementation did not have a significant effect on the jejunal microflora in a group of dogs with naturally occurring EPI;50 however, a study completed in dogs with experimentally induced EPI revealed that clinical signs improved with pancreatic enzyme supplementation alone.51 Administration of tylosin (20-40 mg/kg bid for 2-3 weeks), oral oxytetracycline (10-20 mg/kg bid for 2-3 weeks), or oral metronidazole
(10-20 mg/kg bid for 2-3 weeks) may improve overall response to therapy.
However… PLEASE always consult with your vet be using any antibiotics***
Glucocorticoid therapy
Failure to respond to the above mentioned therapeutic measures warrants consideration of concurrent lymphocytic-plasmacytic gastroenteritis, which if confirmed via gastrointestinal biopsy, may resolve with concurrent prednisone therapy.
References
1. Guilford WG: Nutritional management of gastrointestinal diseases. In Guilford WG, Center SA, Strombeck DR, Williams DA and Meyer DJ, eds.: Strombeck’s Small Animal Gastroenterology. Third Ed. Philadelphia: WB Saunders, 1996, pp 889-910.
2. Guilford WG: Personal communication, Davis, CA, 1999. 3. Foster L.A, Hoskinson J.J, Goggin J.M, and Butine M.D. Gastric emptying of diets varying in micronutrient composition in cats. Proceedings,
1998 Purina Nutrition Forum, p 61).
4. Lin HC, Doty JE, Reedy TJ, et al: Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed by nutrient. Am J Physiol 259:G1031-1036, 1990.
5. Meyer JH, Elashoff JD, Domeck M, et al. Control of canine gastric emptying of fat by lipolytic products. Am J Physiol 266:G1017-1035, 1994.
6. Hofmann AF, Poley JR: Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection: I. Response to cholestyramine or replacement of dietary long chain
triglyceride by medium chain triglyceride. Gastroenterology 62:918- 934, 1972.
7. Cummings JH, Wiggins HS, Jenkins DJA, et al: Influence of diets high and low in animal fat on bowel habit, gastrointestinal transit time, fecal microflora, bile acid, and fat excretion. J Clin Invest
61:953-963, 1978.
8. Guilford WG, Jones BR, Markwell PJ, Arthur DG, Collett MG and Harte JG. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. JVIM 15: 7-13, 2001.
9. Nelson RW, Stookey LJ, Kazacos E. Nutritional management of idiopathic chronic colitis in the dog. JVIM 2:133-137, 1988.
10. Nelson RW, Dimperio ME, Long GG. Lymphocytic-plasmacytic colitis in the cat. JAVMA 184: 1133-1135, 1984.
11. Paterson S. Food hypersensitivity in 20 dogs with gastrointestinal signs. JSAP 36:529-534, 1995.
12. Guilford WG: Idiopathic inflammatory bowel diseases. In Guilford WG, Center SA, Strombeck DR, Williams DA and Meyer DJ, eds.: Strombeck’s Small Animal Gastroenterology. Third Ed. Philadelphia:
WB Saunders, 1996, pp 451-486.
13. Mansfield JC, Giaffer MH, Holdsworth CD: Controlled diet of oligopeptide versus amino acid diet in treatment of active Crohn’s disease.Gut 36:60-66, 1995.
14. Casellas F, Agaude S, Soriano B, et al: Intestinal permeability to 99mTc-diethylenetriaminopentaacetic acid in inflammatory bowel disease. Am J Gastroenterol 81:767-770, 1986.
15. Mayberry JF, Rhodes J, Heatley RV: Infections which cause ileocolic disease in animals: Are they relevant to Crohn’s disease? Gastroenterology 78:1080-1084, 1980.
16. Belsheim MR, Darwish RZ,Watson WC, et al: Bacterial L-form isolation from inflammatory bowel disease patients. Gastroenterology 85:364-369, 1983.
17. Giaffer MH, Cann P, Holdsworth CD: Long-term effects of elemental and exclusion diets for Crohn’s disease. Aliment Pharmacol Ther 5:115-125, 1988.
18. Batt RM, McLean L, Riley JE: Response of the jejunal mucosa of dogs with aerobic and anaerobic bacterial overgrowth to antibiotic therapy. Gut 29:473-482, 1988.
19. Sartor RB: Microbial factors in the pathogenesis of Crohn’s disease, ulcerative colitis and experimental intestinal inflammation, in Kirsner JB, Shorter RG (eds): Inflammatory bowel disease. Baltimore, MD,
Williams & Wilkins, 1995, pp 96-124.
20. Leib MS, Hiler LA, Thatcher C, et al: Plasmacytic lymphocytic colitis in the dog. Sem Vet Med Surg 4:241-246, 1989.
21. Simpson JW, Maskell IE, Markwell PJ: Use of a restricted antigen diet in the management of idiopathic canine colitis. J Small Anim Prac 35:233-238, 1994.
22. Willard MD: Dietary therapy in large intestinal diseases. Proc 6th ACVIM 713, 1988.
23. Marks SL. Management of canine inflammatory bowel disease. Comp Cont Ed 20: 317-332, 1998.
24. Sparkes AH, Papasouliotis K, Sunvold G et al. Bacterial flora in the duodenum of healthy cats and effect of dietary supplementation with fructooligosaccharides. AJVR 59: 431-435, 1998.
25. Sparkes AH, Papasouliotis K, Sunvold G et al. Effect of dietary supplementation with fructooligosaccharides on fecal flora of healthy cats. AJVR 59: 436-440, 1998.
26. Willard MD, Simpson RB, Delles EK et al. Effects of dietary supplementation of fructooligosaccharides on small intestinal bacterial overgrowth in dogs. AJVR 55:654-659, 1994.
27. Leib MS. Treatment of chronic idiopathic large bowel diarrhea in dogs with a highly digestible diet and soluble fiber: A retrospective review of 37 cases. JVIM 14: 27-32, 2000.
28. Hawthorne AB, Edwards T, Filopowicz B et al. Fish oil modifies neutrophil (PMN) function in ulcerative colitis. Gut A738, 1989.
29. Scheurlen M, Dais W, Steinhilber D et al. Effects of long-term application of fish oil on patients with Crohn’s disease. Scand J Gastroenterol uppl 158; 100-101, 1989.
30. Hawthorne AB, Daneshmend TK, Hawkey CJ et al. Treatment of ulcerative colitis with fish oil supplementation: A prospective 12- month randomized controlled trial. Gut 33: 922-928, 1992.
31. Girelli D, Olivieri O, Stanzial AM et al. Factors affecting the thibarbituric acid test as index of red blood cell susceptibility to lipid peroxidation: a mulitvariate analysis. Clin Chim Acta 227: 45-57, 1994.
32. Vaughn DM, Reinhart GA, Swaim SF, et al. Evaluation of effects of dietary n-6 to n-3 fatty acid ratios on leukotriene B synthesis in dog skin and neutrophils. Vet Derm 5: 163, 1994.
33. Reinhart GA, Vaughn DM. Dietary fatty acid ratios and tissue fatty acid content. Proc 13th ACVIM Forum, Lake Buena Vista, FL, 1995: 466-469.
34. Ohlsson K, Ganrot PO, Laurell CB: In vivo interaction between trypsin and some plasma proteins in relation to tolerance to intravenous infusion of trypsin in dogs. Acta Chir Scand 137:113-121, 1971.
35. Leese T, Holliday M, Heath D, et al: Multicentre clinical trial of low volume fresh frozen plasma therapy in acute pancreatitis. Br J Surg 74:907-911, 1987.
36. Williams DA. Personal communication, Florida, 1989.
37. Akol K, Washabau R, Saunders H, et al. Acute Pancreatitis in Cats with hepatic Lipidosis. J Vet Intern Med 7:205-209, 1993.
38. Hill R, Van Winkle T. Acute Necrotizing Pancreatitis and Acute Suppurative Pancreatitis in the Cat: a retrospective study of 40 cases (1976-1989). J Vet Intern Med 7:25-33, 1993.
39. Williams DA: The pancreas. In Guilford WG, Center SA, Strombeck DR, Williams DA and Meyer DJ, eds.: Strombeck’s Small Animal Gastroenterology. Third Ed. Philadelphia: WB Saunders, 1996, p 381.
40. Westermarck E, Junttila J, Wiberg M. The role of low dietary fat in the treatment of dogs with exocrine pancreatic insufficiency. Am J VetRes 56:600-605, 1995.
41. Suzuki A, Mizumoto A, rerknimitr R, et al. Effect of bacterial or porcine lipase with low- or high-fat diets on nutrient absorption in pancreatic- insufficient dogs. Gastroenterology 116:431-437, 1999.
42. Isaksson G, Lundquist I, Akesson B, et al. Effects of pectin and wheat bran in intraluminal pancreatic enzyme activities and on fat absorption as examined with the triolein breath test in patients with pancreatic insufficiency. Scand J Gastroenterol 19:467-472, 1983.
43. Pidgeon G, Strombeck DR. Evaluation of treatment for pancreatic exocrine insufficiency in dogs with ligated pancreatic ducts. Am J Vet Res 43:461-464, 1982.
44. Wiberg ME, Lautala HM, Westermarck E. Rrespnse to long-term enzyme replacement treatment in dogs with exocrine pancreatic insufficiency. JAVMA 213:86-90, 1998.
45. Batt RM, Morgan JO. Role of serum folate and vitamin B12 concentrations in differentiation of small intestinal abnormalities in the dog. Res Vet Sci 32:17-22, 1982.
46. Batt RM, McLean L, Rutgers HC, Hall EJ. Validation of a radioassay for the determination of serum folate and cobalamin concentrations in dogs. J Small Anim Pract 32:221-224, 1991.
47. Simpson KW, Morton DB, Batt RM. Effect of exocrine pancreatic insufficiency on cobalamin absorption in dogs. Am J Vet Res 50:1233-1236, 1989.
48. Allen RH, Stabler SP, Savage DG, Lindenbaum J. Metabolic abnormalities
in cobalamin (vitamin B12) and folate deficiency. FASEB J7:1344-1353, 1993.
49. Simpson KW, Fyfe J, Cornetta A, et al. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med 15:26-32, 2001.
50. Westermarck E, Myllys V, Aho M. Effect of treatment on the jejunal and colonic bacterial flora of dogs with exocrine pancreatic insufficiency. Pancreas 8:559-562, 1993.
51. Simpson KW, Batt RM, Jones D, et al. Effects of exocrine pancreatic insufficiency and replacement therapy on the bacterial flora of the duodenum in dogs. Am J Vet Res 51:203-206, 1990.
PRINCIPLES IN THE THERAPY OF CANINE INFLAMMATORY BOWEL DISEASE 2009
Robert J. Washabau, VMD, PhD, Dipl. ACVIM – [email protected]
Professor of Medicine and Department Chair
Department of Veteirnary Clinical Sciences
College of Veterinary Medicine, 1352 Boyd Avenue
University of Minnesota, St. Paul, Minnesota 55108
IBD has been defined clinically as a spectrum of gastrointestinal disorders associated with chronic
inflammation of the stomach, intestine and/or colon of unknown etiology. Management of IBD
consists of 1) dietary therapy, 2) exercise, 3) antibiotics, 4) probiotics, 5) anti-diarrheal agents, 6)
restoration of normal motility, 7) anti-inflammatory or immunosuppressive therapy, and 8)
behavioral modification.
1. Dietary Therapy
The precise immunologic mechanisms of canine and feline IBD have not yet been determined, but
a prevailing hypothesis for the development of IBD is the loss of immunologic tolerance to the
normal bacterial flora or food antigens. Accordingly, dietary modification may prove useful in the
management of canine and feline IBD. Several nutritional strategies have been proposed including
novel proteins, hydrolyzed diets, anti-oxidant diets, medium chain triglyceride supplementation,
low fat diets, modifications in the omega-6/omega-3 (ω-6/ω-3) fatty acid ratio, and fiber
supplementation. Of these strategies, some evidence-based medicine has emerged for the use of
novel protein, hydrolyzed, anti-oxidant-supplemented diets, and prebiotics.
2. Exercise
Experimental IBD in the dog is accompanied by significant abnormalities in the normal colonic
motility patterns. Physical exercise has been shown to disrupt the colonic MMCs and to increase
the total duration of contractions that are organized as non-migrating motor complexes during the
fed state. Exercise also induces GMCs, defecation, and mass movement in both the fasted and fed
states. The increased motor activity of the colon and extra GMCs that result from physical
exercise may aid in normal colonic motor function.
3. Antibiotics
Some IBD cases are initiated by true enteric pathogens, while others are complicated by small
intestinal bacterial overgrowth. Some IBD cases may show short term responsiveness to one or
more antibiotics, e.g., tylosin, metronidazole, or oxytetracycline.
4. Probiotics
Probiotics are living organisms with low or no pathogenicity that exert beneficial effects (e.g.,
stimulation of innate and acquired immunity) on the health of the host. The Gram-positive
commensal lactic acid bacteria (e.g., Lactobacilli) have many beneficial health effects, including
enhanced lymphocyte proliferation, innate and acquired immunity, and anti-inflammatory cytokine
production. Lactobacillus rhamnosus GG, a bacterium used in the production of yogurt, is effective
in preventing and treating diarrhea, recurrent Clostridia difficile infection, primary rotavirus
infection, and atopic dermatitis in humans. Lactobacillus rhamnosus GG has been safely colonized
in the canine gastrointestinal tract, although probiotic effects in the canine intestine have not been
firmly established. The probiotic organism, Enterococcus faecium (SF68), has been safely colonized
in the canine gastrointestinal tract, and it has been shown to increase fecal IgA content and
circulating mature B (CD21+/MHC class II+) cells in young puppies. It has been suggested that
this probiotic may be useful in the prevention or treatment of canine gastrointestinal disease. This
organism may, however, enhance Campylobacter jejuni adhesion and colonization of the dog
intestine, perhaps conferring carrier status on colonized dogs.
5. Anti-Diarrheal Agents
Prostaglandin Synthetase Inhibitors
– Sulfasalazine – 10-25 mg/kg TID-QID, PO
– 5-aminosalicylate – 5-10 mg/kg PO, TID-QID (dog)
μ,δ-Opioid Agonists
– Loperamide 0.08 mg/kg TID, PO-preferred drug
5-HT3 Serotonin Antagonists
– Ondansetron (Zofran, Glaxo) – 0.5-1.0 mg/kg BID, PO
– Granisetron (Kytril, SmithKline Beecham) – 0.5-1.0 mg/kg BID, PO
α2-Adrenergic Antagonists – These drugs must be used carefully as they can activate α2-adrenergic
receptors in the chemoreceptor trigger zone and cause vomiting.
– Clonidine 5-10 μg/kg BID-TID, SQ/PO
6. Restoration of Normal Motility
The mixed μ,δ-opioid agonist, loperamide, stimulates colonic fluid and electrolyte absorption while
inhibiting colonic propulsive motility. Loperamide (0.08 mg/kg PO TID-QID) may be beneficial in
the treatment of difficult or refractory cases of large bowel-type IBD.
7. Anti-Inflammatory/Immunosuppressive Therapy
Sulfasalazine – Sulfasalazine is a highly effective prostaglandin synthetase inhibitor that has
proven efficacy in the therapy of large bowel IBD in the dog. Sulfasalazine is a compound
molecule of 5-aminosalicylate (meselamine) and sulfapyridine linked in an azo chemical bond.
Following oral dosing, most of the sulfasalazine is transported to the distal gastrointestinal tract
where cecal and colonic bacteria metabolize the drug to its component parts. Sulfapyridine is
largely absorbed by the colonic mucosa but much of the 5-aminosalicylate remains in the colonic
lumen where it inhibits mucosal lipoxygenase and the inflammatory cascade. Sulfasalazine has
been recommended for the treatment of canine large bowel IBD at doses of 10-25 mg/kg PO TID
for 4-6 weeks. With resolution of clinical signs, sulfasalazine dosages are gradually decreased by
25 per cent at 2-week intervals and eventually discontinued while maintaining dietary
management. Salicylates are readily absorbed and induce toxicity in cats, therefore this drug
classification should be used with great caution in cats. If used in cats, some authors have
recommended using half of the recommended dog dose (i.e., 5-12.5 mg/kg PO TID. Sulfasalazine
usage has been associated with the development of keratoconjunctivitis sicca in the dog, so tear
production should be assessed subjectively (by the pet owner) and objectively (by the
veterinarian) during usage.
Metronidazole – Metronidazole (10-20 mg/kg PO BID-TID) has been used in the treatment of mild
to moderate cases of large bowel IBD in both dogs and cats. Metronidazole has been used either
as a single agent or in conjunction with 5-aminosalicylates or glucocorticoids. Metronidazole is
believed to have several beneficial properties, including anti-bacterial, anti-protozoal, and
immunomodulatory effects. Side effects include anorexia, hypersalivation, and vomiting at
recommended doses and neurotoxicity (ataxia, nystagmus, head title, and seizures) at higher
doses. Side effects usually resolve with discontinuation of therapy but diazepam may accelerate
recovery of individual patients.
Glucocorticoids – Anti-inflammatory doses of prednisone or prednisolone (1-2 mg/kg PO SID) may
be used to treat IBD in dogs that have failed to respond to dietary management, sulfasalazine, or
metronidazole, and as adjunctive therapy to dietary modification in feline IBD. Prednisone or
prednisolone is used most frequently, as both have short durations of action, are cost-effective,
and are widely available. Equipotent doses of dexamethasone are equally effective but may have
more deleterious effects on brush border enzyme activity. Prednisone should be used for 2-4
weeks depending upon the severity of the clinical signs. Higher doses of prednisone (e.g., 2-4
mg/kg PO SID) may be needed to control severe forms of eosinophilic colitis or hypereosinophilic
syndrome in cats. Combination therapy with sulfasalazine, metronidazole, or azothioprine may
reduce the overall dosage of prednisone needed to achieve remission of clinical signs. As with
sulfasalazine, the dose of glucocorticoid may be reduced by 25% at 1-2 week intervals while
hopefully maintaining remission with dietary modification. Because of steroid side effects and
suppression of the hypothalamic-pituitary-adrenal axis, several alternative glucocorticoids have
been developed that have excellent topical (i.e., mucosal) anti-inflammatory activity but are
significantly metabolized during first pass hepatic metabolism. Budesonide has been used for
many years as an inhaled medication for asthma, and an enteric-coated form of the drug is now
available for treatment of IBD in humans (and animals). There is little evidence-based medicine in
support of the use of this medication in canine or feline IBD, but doses of 1 mg/cat or 1 mg/dog
per day have been used with some success in anecdotal cases.
8. Behavioral Modification
Inflammatory bowel disease and irritable bowel syndrome very likely have underlying behavioral
components. Abnormal personality traits and potential environmental stress factors were
identified in 38% of dogs in one study. Multiple factors were present in affected households,
including travel, re-location, house construction, separation anxiety, submissive urination, noise
sensitivity, and aggression. The role of behavior in the pathogenesis and therapy of canine and
feline gastrointestinal disorders remains largely unexplored.
HOW I TREAT INFLAMMATORY BOWEL DISEASE IN DOGS
Stanley L. Marks, BVSc, PhD, DACVIM (Internal Medicine, Oncology), DACVN University of California,
Davis, School of Veterinary Medicine, Davis, CA, USA
Professor of Small Animal Medicine
34th World Small Animal Veterinary Congress 2009 – São Paulo, Brazil
The inflammatory bowel diseases (IBD) are the most common causes of chronic vomiting and diarrhea
in dogs and cats, and refer to a group of poorly understood enteropathies characterized by the
infiltration of the gastrointestinal mucosa by inflammatory cells.1 The cellular infiltrate is composed of
variable populations of lymphocytes, plasma cells, eosinophils, macrophages, neutrophils, or
combinations of these cells. Changes in the mucosal architecture characterized by villous atrophy,
fusion, fibrosis, and lacteal dilation frequently accompany the cellular infiltrates.
Although the etiology of canine IBD is poorly understood, there is provocative evidence from clinical
observations and animal models to incriminate normal luminal bacteria or bacterial products in the
initiation and perpetuation of canine and feline IBD. Evidence of the role of enteric microflora in the
pathogenesis of IBD in people is supported by clinical responses to fecal stream diversion treatment in
patients with Crohn’s disease (CD)2 and antimicrobial therapy in CD and ulcerative colitis (UC)
patients.3 Additionally, there are increases in circulating and intraluminal humoral and T-cell
responses to the enteric microflora in human IBD patients. Furthermore, genetic mutations in
NOD2/CARD154 and TLR-4 (Toll-like-receptor-4) in IBD patients make them less able to detect
bacterial components, resulting in defective responses to enteric microflora.5 Studying the
composition of the intestinal microflora has been a challenge to researchers; however, recent work
has focused closely on the bacteria associated with the mucosal lining. A study of adherent mucosal
bacteria in IBD patients concluded that Bacteroides fragilis comprised >60% of the biofilm mass in
patients with IBD.6 Dietary factors also appear to play a role in the etiopathogenesis of IBD in dogs
and cats based on the clinical response to elimination or “hypoallergenic” diets in many of these
animals.
MANAGEMENT OF IBD
PRINCIPLES OF NUTRITIONAL MANAGEMENT
Elimination/Novel Protein Diets
Antigenic determinants on proteins are incriminated in many cases of IBD, implying that the
feeding of select protein diets containing a single, highly digestible, novel protein source might be
beneficial for managing dogs and cats with IBD.9
Hypoallergenic diets
The ability to induce an antibody mediated hypersensitivity response appears to be dependent
upon the size and structure of the protein. The allergens in soybean protein, for example, are
between 20 and 78 kilodaltons, suggesting that soybean proteins with a molecular weight below this
threshold would be less likely to illicit an immune-mediated response. Hypoallergenic diets are
particularly beneficial as elimination diets for the diagnosis and management of food hypersensitivity,
when a patient appears to be allergic to multiple allergens, when a complicated dietary history makes
it difficult to identify a “novel” protein, or when a patient has severe IBD.10
Dietary Fiber
The gelling and binding properties of fatty acids and deconjugated bile acids in soluble fibers
may be beneficial in certain gastrointestinal diseases. The use of soluble (fermentable) fiber in
preference to insoluble (non-fermentable) fiber is generally advocated because most soluble fibers
generate butyrate, the principle source of energy for the colonocyte, and other short-chain fatty acids.
Short-chain fatty acids may lower the colonic luminal pH, impeding the growth of pathogens.11 The
health benefits derived from dietary supplementation of prebiotics have been documented in humans
and feeding oligofructose to dogs decreased the concentrations of fecal ammonia and amines and
increased the numbers of bifidobacteria in dog feces.12
Polyunsaturated fatty acids
Fish oil has been reported to be beneficial in ulcerative colitis and Crohn’s disease patients,13
but the results are controversial. Only a few studies found significant decreases in rectal LTB4
concentrations; the others simply reported clinical improvement. There are no published studies in
the veterinary literature to date demonstrating the efficacy of n-3 fatty acid supplementation in
managing canine or feline patients with IBD.
Fat
Avoiding excessive fat can be instrumental in the management of various gastrointestinal
diseases because fat delays gastric emptying in dogs and high-fat foods may contribute to osmotic
diarrhea. Malabsorbed fatty acids are hydroxylated by intestinal bacteria and stimulate colonic water
secretion, exacerbating diarrhea as well as gastrointestinal protein and fluid losses.14
Vitamins and Minerals
Water-soluble vitamins are often depleted by the fluid losses associated with diarrhea and fatsoluble
vitamin loss can be significant in animals with steatorrhoea. Magnesium deficiency has been
well documented in Yorkshire Terriers with severe inflammatory bowel disease and
lymphangiectasia.15 Cats with severe IBD frequently have subnormal serum cobalamin concentrations.
Patients with mild-to-moderate IBD can often be successfully managed with dietary
modification and antimicrobial (tylosin or metronidazole) administration. Dogs and cats with lack of
response to more conservative therapy or patients with severe IBD based on activity index scores or
histologic findings should be managed with immunomodulatory therapy.
PHARMACOLOGIC MANAGEMENT
Most dogs and cats with moderate to severe IBD will require adjuvant pharmacologic therapy
in combination with dietary management. It is important to understand that the therapy of IBD must
be tailored according to each patient’s response.
Oral Corticosteroids
Corticosteroids remain the cornerstone of medical therapy for IBD, despite the lack of
published controlled clinical trials documenting their benefit in dogs with IBD. The value of
corticosteroids relates to their anti-inflammatory and immunosuppressive properties, although they
also increase intestinal sodium and water absorption in the small and large bowel, and regulate basal
colonic electrolyte transport. The dosage and duration of therapy is based on the severity and duration
of clinical signs, the severity and type of inflammation, the clinical response, and tolerance to the
drug. The initial dosage of prednisone for therapy of IBD in dogs is 1 to 2 mg/kg q 12 hours. The drug
is gradually tapered over a 6- to 10-week period once clinical remission is attained. Combination
therapy with dietary therapy, azathioprine, or metronidazole is undertaken with the goal of reducing
the dose of prednisone. Parenteral corticosteroid therapy is reserved for vomiting patients, or animals
with severe non-responsive disease.
Budesonide, an orally administered corticosteroid structurally related to 16-
hydroxyprednisolone, has high topical anti-inflammatory activity and low systemic activity because of
its high affinity to the steroid receptor and rapid hepatic conversion to metabolites with minimal or no
steroid activity. The drug is dosed at 1 mg once daily for toy-breed dogs, and up to 2 mg BID for
large or giant breed dogs.
Azathioprine
Azathioprine is an antimetabolite that is converted to 6-mercaptopurine in the liver and then
to thioinosinic acid. The latter compound impairs purine biosynthesis and this biochemical reaction
inhibits cellular proliferation and reduces natural killer cell cytotoxicity.16 The onset of these
immunological effects is slow, and can require several months for maximal effectiveness. The drug is
most useful in dogs as adjunctive therapy in severe or refractory IBD. Azathioprine can also be used
for its steroid-sparing effects when the adverse effects of prednisone are unacceptably high. The dose
for dogs is 50 mg/m2 or 1-2 mg/kg once daily for 2 weeks, followed by alternate-day administration.
Side-effects of the dug in dogs include anorexia, pancreatitis, and hepatic dysfunction.
Chlorambucil
The alkylating agent chlorambucil is beneficial for managing refractory cases of IBD,
particularly in cats. Hematological monitoring is warranted every 3-4 weeks to assess for
neutropenia. Chlorambucil can be administered at 15 mg PO/m2 once per day for 4 consecutive days,
and repeated q 3 weeks (in combination with prednisone) or administered at 2 mg per cat q 4 days
indefinitely. In dogs chlorambucil is administered at 1.5 mg/m2 every alternate day.
Cyclosporine
Cyclosporine has been demonstrated to be effective in dogs with IBD that were refractory to
immunosuppressive doses of prednisone.17 The dose of cyclosporine used was 5 mg/kg q 24 hrs and
the drug was well tolerated.
Sulfasalazine
The drug consists of sulfapyridine linked to mesalamine (previously called 5-aminosalicylic
acid) by an azo bond that is cleaved by colonic bacteria with subsequent release of the active moiety
of the drug, mesalamine. Sulfapyridine is almost completely absorbed in the colon, metabolized in the
liver, and excreted in the urine. The mesalamine moiety is locally absorbed and inhibits the formation
and degradation of inflammatory mediators, including leukotrienes, prostaglandins, thromboxane,
platelet activating factor, histamine, and a number of cytokines. Sulfasalazine is of no value in
managing small bowel inflammation because colonic bacterial metabolism is needed to release the
active moiety. The usual initial dose in dogs is 20 to 40 mg/kg q 8 hours for 3 weeks, followed by 20
to 40 mg/kg q 12 hours for 3 weeks, and 10 to 20 mg/kg q 12 hours for 3 weeks. The most common
side-effects of sulfasalazine include anorexia, vomiting, cholestatic jaundice, allergic dermatitis, and
keratoconjunctivitis sicca (KCS).
Antimicrobials
Metronidazole (Flagyl), an inhibitor of cell-mediated immunity,18 has been frequently used as
an adjunctive agent for the management of IBD. The dose of metronidazole is 10 to 15 mg/kg q 8 to
12 hours. Metronidazole tablets have a sharp, unpleasant, metallic taste when scored that can cause
severe salivation. Side-effects are rare, although metronidazole has been associated with a peripheral
neuropathy in humans and animals. Less common side effects include inappetence, nausea, vomiting,
seizures, and reversible neutropenia. Tylosin (Tylan) is a macrolide antibiotic that has been reported
to be effective and safe in managing canine IBD and antibiotic responsive diarrhea (ARD).19 Although
the drug’s mechanism of action is unknown, it appears to be effective in some dogs’ refractory to
other forms of therapy. The dose range is 20 to 40 mg/kg q 12 hours.
Probiotics
Administration of probiotics to dogs and cats with IBD represents a novel alternative
therapeutic modality that warrants further investigation. It has been demonstrated that colitis in both
humans and mice is associated with increased levels of cytokines such as TNF-α, IL-6, IL-12p70 and
IL-23.20,21 Thus, a proper selection of probiotic strains for the treatment of IBD is crucial and should
be based on the estimation of their capacity to induce anti-inflammatory pattern of cytokines (IL-
10high, TGF-βhigh, IL-12p70low, IL-23low, TNF-αlow). Apart from immunomodulatory effects, probiotics
have a protective effect on the normal microflora of the human gut by their antimicrobial activities
directed toward intestinal pathogens.22
Probiotics have also been utilized to facilitate eradication of intestinal parasites. A recent
study documented the ability of the probiotic organism Enterococcus faecium SF68 (FortiFlora, Nestle-
Purina, St. Louis, MO) to antagonize Giardia intestinalis infection in mice.23 Oral feeding of E. faecium
strain SF68 starting 7 d before inoculation with Giardia trophozoites significantly increased the
production of specific anti-Giardia intestinal IgA and blood IgG. This humoral response was mirrored at
the cellular level by an increased percentage of CD4(+) T cells in the Peyer’s patches and in the
spleens of SF68-fed mice. The improvement of specific immune responses in probiotic-fed mice was
associated with a diminution in the number of active trophozoites in the small intestine as well as
decreased shedding of fecal Giardia antigens (GSA65 protein).
References
1. Guilford WG: Idiopathic inflammatory bowel diseases, in Guilford WG, Center SA,
Strombeck DR, Williams DA, Meyer DJ (eds): Strombeck’s Small Animal Gastroenterology.
Third Ed., 1996, pp 451-486.
2. Winslet MC, et al. Fecal diversion for Crohn’s colitis: a model to study the role
of the fecal stream in the inflammatory process Gut 1994;35:236-242.
3. Gionchetti P, et al. Antibiotics and probiotics in treatment of inflammatory bowel
disease. World J Gastroenterol 2006;12:3306-3313.
4. Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to
Crohn’s disease. Nature 2001;411:599-603.
5. Franchimont D, et al. Deficient host-bacteria interactions in inflammatory bowel
disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s
disease and ulcerative colitis. Gut 2004;53:987-992.
6. Swidsinski A, et al. Mucosal flora in inflammatory bowel disease.
Gastroenterology 2002;122: 44-54.
7. German AJ, et al. Comparison of direct and indirect tests for small intestinal bacterial
overgrowth and antibiotic-responsive diarrhea in dogs. J Vet Intern Med 2003;17(1):33-43.
8. Willard MD, et al. Interobserver variation among histopathologic evaluations of
intestinal tissues from dogs and cats. J Am Vet Med Assoc 2002;15;220(8):1177-82.
9. Guilford WG, et al. Food sensitivity in cats with chronic idiopathic gastrointestinal
problems. J Vet Int Med 2001;15:7-13.
10. Marks SL, et al. Dietary trial using a commercial hypoallergenic diet containing
hydrolyzed protein for dogs with inflammatory bowel disease Vet Therapeutics 2002;3:109-
118.
11. Brockett M, Tannock GW. Dietary influence on microbial activities in the cecum of
mice. Can J Microbiol 1982;28:493-499.
12. Hussein HS, et al. Petfood applications of inulin and oligofructose. J Nutr 1999;129(7
Suppl):1454S-6S
13. Seidner DL, et al. An oral supplement enriched with fish oil, soluble fiber, and
antioxidants for corticosteroid sparing in ulcerative colitis: a randomized, controlled trial.
Clin Gastroenterol Hepatol. 2005 Apr;3(4):358-69.
14. Cummings JH, et al: Influence of diets high and low in animal fat on bowel habit,
gastrointestinal transit time, fecal microflora, bile acid, and fat excretion. J Clin Invest
1978;61:953-963.
15. Kimmel SE, et al. Hypomagnesemia and hypocalcemia associated with protein-losing
enteropathy in Yorkshire terriers: five cases (1992-1998). J Am Vet Med Assoc
2000;1;217(5):703-6.
16. Brogan M, et al: The effect of 6-mercaptopurine on natural killer-cell activities in
Crohn’s disease. J Clin Immunol 1985;5:204-211.
17. Allenspach K, et al. Pharmacokinetics and clinical efficacy of cyclosporine treatment of
dogs with steroid-refractory inflammatory bowel disease. J Vet Intern Med 2006;20(2):239-
44.
18. Grove DI. Suppression of cell-mediated immunity by metronidazole. Int Arch Allergy
Appl Immunol 1977;54(5):422-7.
19. Westermarck E, et al. Tylosin-responsive chronic diarrhea in dogs. J Vet Intern Med
2005;19(2):177-86.
20. Becker C., Dornhoff H., Neufert C. et al. Cutting edge: IL-23 cross-regulates IL-12
production in T cell-dependent experimental colitis. J. Immunol 2006;177, 2760–2764.
21. Fuss I.J., Becker C., Yang Z. et al. Both IL-12p70 and IL-23 are synthesized during
active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal
antibody. Inflamm Bowel Dis 2006;12, 9–15.
Reprinted in IVIS with the permission of WSAVA Close this window to return to IVIS
34th World Small Animal Veterinary Congress 2009 – São Paulo, Brazil

http://www.nutriscan.org/knowledge-center/food-sensitivities.html
If you suspect a food sensitivity (which is different from food allergies!) please feel free to check into the Nutriscan testing. We have had a few Epi4Dogs members do this test with dogs that just couldn’t get their SID/SIBO under control and deduced that it was food-related. When they followed the food sensitivity recommended diet after the testing was done…… very positive results were reported. Please discuss with your vet as this might be an option worth looking into.
NutriScan Peer-Reviewed AHVMA winter 2017 Report: Dodds-AHVMA_Winter_2017._2018_Volume_49

| Pathogenesis of canine IBD – What innate immunity can teach us Karin Allenspach Dr Med Vet, FVH, Dipl ECVIM-CA, PhD, FHEA, Hatfield, UKEach day the intestinal mucosa is challenged with a huge array of antigens, either from food or the microbial flora in the intestinal lumen. The gastrointestinal immune system must maintain hypo-responsiveness to these, in order to avoid unwanted and damaging inflammation or autoimmune diseases. However, it must also be able to respond rapidly to the presence of pathogenic bacteria in the gut lumen and to mount an immune response to eradicate the pathogen. How does the intestinal epithelium cope with such challenges? The gut epithelium detects the presence of both commensal and pathogenic bacteria via Pattern Recognition Receptors (PRRs)1. This group contains Toll-like receptors (TLRs) and Nucleotide Binding Oligomerisation Domain-like receptors (NODs)2. These receptors recognize specific molecules termed Pathogen-Associated Molecular Patterns (PAMPs), which are conserved molecules found on bacteria and other infectious agents. Recognition of PAMPs by TLRs is a crucial part of the innate immune response to invading bacteria in the gut and initiates a complex intracellular signalling pathway culminating in the activation of the transcription factor NF-κ B3. This results in the transcription and secretion of a variety of pro- and anti-inflammatory cytokines and chemokines from the cell bearing the TLR. If successful, immune cells activated by this response clear offending pathogens. Despite the fact that commensal and pathogenic bacteria share PAMP, the immune system remains unresponsive towards commensal organisms present in the intestinal lumen in the normal case scenario4. The inflammatory response which is normally only seen as a reaction towards pathogenic bacteria breaching the intestinal barrier is similar to the response seen in the mucosa of dogs affected with IBD. However, the response in IBD occurs in the absence of pathogens. It is believed that the innate immune system reacts to normal commensals in the intestinal lumen as if they were pathogens. TLR-related functional differences definitely play a role in the pathogenesis of IBD in human beings5. Of even bigger interest is that genetic variations in TLRs have been demonstrated and associated with IBD in humans: An Asp299Gly polymorphism in human TLR4 is associated with impaired LPS signalling and an increased susceptibility to gram-negative infections6. This suggests a role for this polymorphism in triggering IBD: The normal function of this receptor is altered, leading to a chronic inflammatory state which is uncontrollable by the host7. Similarly to the human system, IBD in dogs may also be |
Where to go from here? We are still a long way from our goal to try and find better ways of diagnosing and treating chronic enteropathies in dogs. The next step will involve investigations into the functional aspects of canine TLRs. In order to achieve this, current studies at the RVC are investigating signalling through canine TLRs on a cellular level. These investigations will allow insights into the functional consequences of TLR2 polymorphisms observed in German Shepherd Dogs, and will help elucidating the possible pathogenesis of ARD and IBD. The knowledge gained in these investigations will very likely identify potential new treatment strategies for canine IBD. If bacteria or bacterial products are identified which either stimulate the appropriate TLR response or inhibit an aberrant TLR-dependent NF-κ B activation, then this knowledge can be used in prospective treatment studies in dogs with chronic enteropathies. Similar to the human system, it is possible that certain probiotic cocktails could be used as prophylactic treatments in dogs which are genetically predisposed to developing IBD, food allergy or ARD. The identification of a specific bacterial product that reduces inflammation in the gut could serve as a treatment supplement or even as an adjuvant for potential vaccines against IBD in dogs. References 1. Sandor, F. and M. Buc, Toll-like receptors. III. Biological significance |